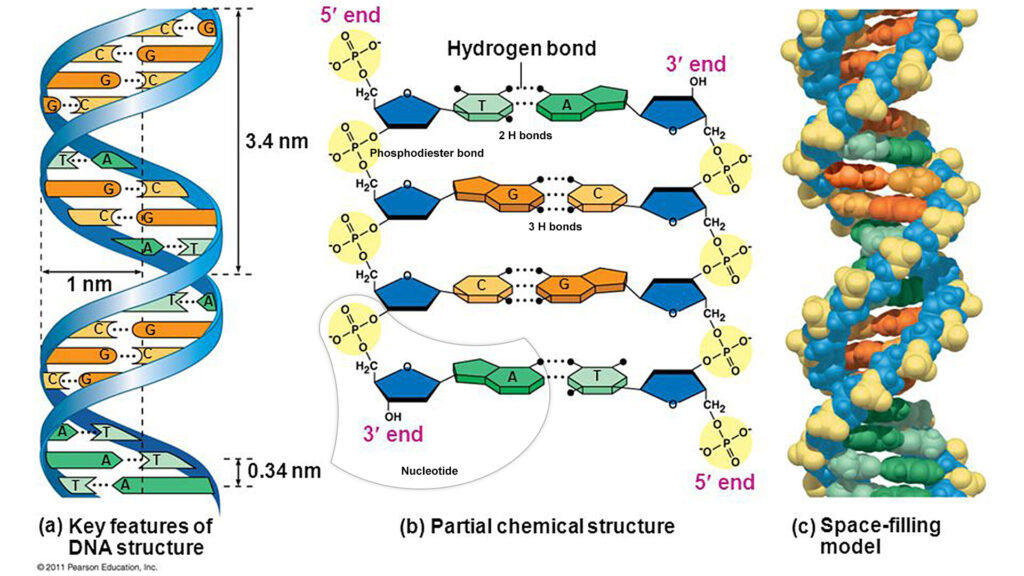
QUESTION: In nucleic acids which bonds are stronger? Phosphodiester or hydrogen?
ANSWER: In the DNA molecule there are two strands or strands of nucleotides forming a double helix (what is helical is coiled on itself, like a spring). They are joined by PHOSPHODIESTER BRIDGES, between the phosphate of one nucleotide and carbon 3 of the pentose of the next nucleotide, and this occurs on both strands, on the two sides of the molecule. So what binds the nucleotides of each chain together are these covalent bonds. On the other hand, hydrogen bonds or bonds, which in reality are not legitimate chemical bonds and therefore much WEATHER than phosphodiester bonds (phosphodiester bond), join the nitrogenous bases of the two nucleotide strands, in the center of the molecule, so that it enters ADENINA and TIMINA there are 2 bridges of H and between GUANINA AND CITOSINE there are 3.
If a linear DNA molecule has 100 nucleotides (50 on each strand) there will be 98 phosphodiester bridges in all, 49 on each strand, as there are 49 spaces between those 50. If it is a plasmid (circular bacterial or mitochondrial or chloroplast DNA) there will be 100 bridges, 50 on each side, because the molecule is closed.
Restriction enzymes cut phosphodiester bridges and ligases do the opposite. The helicases break the bridges of H.
mRNA is a single strand of nucleotides and therefore has only phosphodiester bridges and no H bridges.